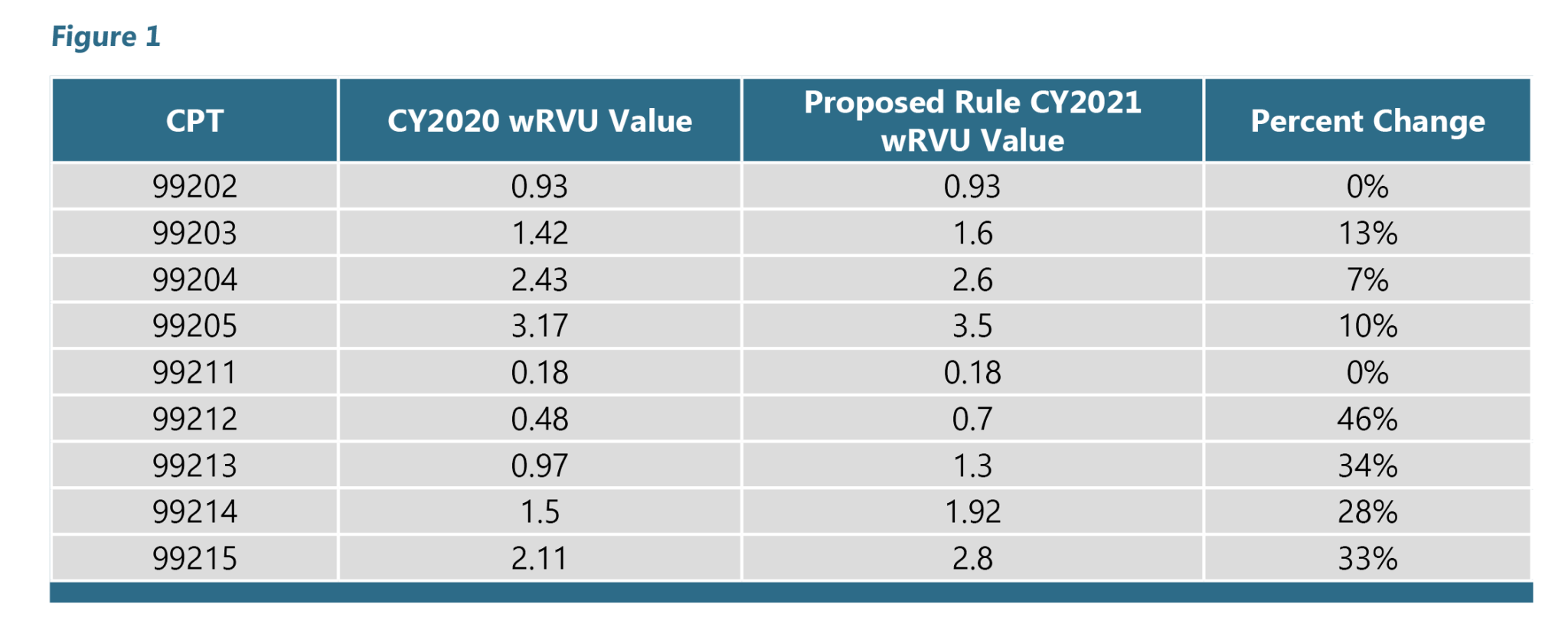
Clinical Laboratory Fee Schedule
| Year for CDLT Rates | Based on Data Reporting Period | Reduction Cap |
| 2020 | January 1, 2017 – May 30, 2017 | 10% |
| 2021 | January 1, 2017 – May 30, 2017 | 0.0% |
| 2022 | January 1, 2017 – March 31, 2017 | 0.0% |
| 2023 | January 1, 2017 – March 31, 2017 | 15% |
Full Answer
How are outpatient clinical laboratory services paid?
Outpatient clinical laboratory services are paid based on a fee schedule in accordance with Section 1833(h) of the Social Security Act. Payment is the lesser of the amount billed, the local fee for a geographic area, or a national limit.
Do co-payments and deductibles apply to the Medicare clinical laboratory fee schedule?
Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule. Each year, new laboratory test codes are added to the clinical laboratory fee schedule and corresponding fees are developed in response to a public comment process.
Does Medicare cover clinical diagnostic laboratory tests?
Note: Including a code and/or payment amount for a particular clinical diagnostic laboratory test does not imply Medicare will cover the test. CY 2021 Q1 Release: Added for January 2021. The update includes all changes identified in CR 12080.
What is the CLFS final rule for clinical lab tests?
The CLFS final rule “Medicare Clinical Diagnostic Laboratory Tests Payment System Final Rule” (CMS-1621-F) was published in the Federal Register on June 23, 2016. The CLFS final rule implemented section 1834A of the Act.
How are outpatient labs paid?
How much is the reduction for CY 2021?
When will CLFS rates be based on PAMA?
How much does a cervical smear cost?
How long is the data reporting cycle for CDLTs?
When is the next data reporting period for CDLTs?
Do co-pays apply to lab fees?
See 4 more
About this website
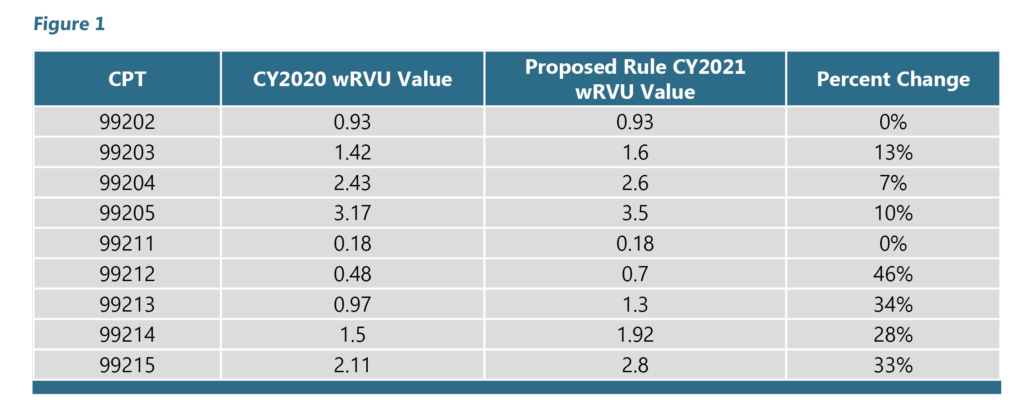
Is clinical laboratory fee schedule cost based or price based?
Outpatient clinical laboratory services are paid based on a fee schedule in accordance with Section 1833(h) of the Social Security Act. Payment is the lesser of the amount billed, the local fee for a geographic area, or a national limit.
Which established the Medicare clinical laboratory fee schedule?
Medicare Part B payments for lab tests are determined by the Clinical Laboratory Fee Schedule. The Deficit Reduction Act of 1984 mandated that fee schedules be established for each lab test on a regional, statewide, or carrier basis.
What is the federal entity that determines laboratory reimbursement fee schedule?
The Centers for Medicare & Medicaid Services (CMS) regulate all laboratory testing and if an entity, including a doctor's office, performs in-vitro diagnostic testing, it is considered a laboratory under CLIA and must register with the CLIA program.
How are labs reimbursed by Medicare?
Medical Part B (Medical Insurance) covers medically necessary clinical diagnostic laboratory tests when your doctor or provider orders them. You usually pay nothing for Medicare-approved clinical diagnostic laboratory tests.
What is gap fill pricing?
Gap-filling is an empirical process of determining a payment amount in a locality using available information sources. Usually the period during which gap-filled payment amounts are instructed is the year following the introduction of a new code.
What is clinical labor pricing?
Clinical labor pricing data are used to calculate Medicare payment rates. This ensures Medicare payments account for the costs of employing nurses, medical assistants, and other clinical staff.
Can labs be billed with modifier 26?
Services with a value of “1” or “6” in the PC/TC Indicator field of the National Physician Fee Schedule may be billed with modifier 26. These are predominantly radiology services, but also include pathology, laboratory and medicine services.
How do you calculate CPT reimbursement rate?
You can search the MPFS on the federal Medicare website to find out the Medicare reimbursement rate for specific services, treatments or devices. Simply enter the HCPCS code and click “Search fees” to view Medicare's reimbursement rate for the given service or item.
What labs are included in CPT 80050?
CPT code 80050, is composed of metabolic panel, a complete blood count and a TSH level.
How are labs billed?
All lab tests are billed via a set of current procedural terminology (CPT) codes, and all the considerations named above require attention for what is a relatively complex billing cycle.
Can you use modifier 59 on labs?
Modifier 59 (distinct) and 91 (repeat) are valid modifiers for most laboratory services and should be used when multiple laboratory services described by a single code are provided to a patient on one day by the same provider. It is important to use the right modifier for the situation.
What is the 70/30 rule for Medicare?
The “70/30 rule” which requires laboratories to perform in-house at least 70 percent of what is billed to Medicare, and refer or send out no more than 30 percent of what is billed to Medicare continues to apply under the demonstration.
Which established the Medicare clinical laboratory fee schedule which is a data set based on local fee schedules for outpatient clinical diagnostic laboratory services?
The Medicare Clinical Diagnostic Laboratory Fee Schedule for outpatient services was established as part of the Deficit Reduction Act of 1984.
What are fee schedules?
A fee schedule is a complete listing of fees used by Medicare to pay doctors or other providers/suppliers. This comprehensive listing of fee maximums is used to reimburse a physician and/or other providers on a fee-for-service basis.
Which is added to DSH or IME adjustments made for hospitals that treat unusually costly cases resulting in increased medical payments?
Hospitals that treat unusually costly cases receive increased medical payments. Outlier payments are added to DSH or IME adjustments, when applicable.
Which is a predetermined reimbursement methodology required for several health care programs administered by the federal government?
A Prospective Payment System (PPS) is a method of reimbursement in which Medicare payment is made based on a predetermined, fixed amount.
Clinical Laboratory Fee Schedule Files | CMS
File Name Description Calendar Year; 22CLABQ2: CY 2022 Q2 Release: Added for April 2022. The update includes all changes identified in CR 12612. The file has 1,874 records.
SE19006 - Medicare Part B Clinical Laboratory Fee Schedule: Revised ...
MLN Matters SE19006 Related CR N/A. Page 2 of 25 Under the CLFS final rule, reporting entities must report to us certain private payor rate information (applicable information) for their component applicable laboratories.
Clinical Laboratory Fee Schedule | Guidance Portal - HHS.gov
For more information regarding the CLFS Data and Reporting for CDLTs, please visit the CMS PAMA webpage.IMPORTANT UPDATE: Next CLFS Data Reporting Period for Clinical Diagnostic Laboratory Tests - DELAYED
Medicare Clinical Laboratory Fee Schedule
and Final 2018 CLFS (Released Nov. 2017) HCPCS/ CPT Code Description 2017 National Limit Amount Weighted Median 2018 Payment w/ Cap Payment % Change
Medicare Clinical Laboratory Fee Schedule - American Medical Association
Data collection period: January 1 to June 30, 2016 . June 23, 2016 . CMS published regulatory requirements • Based on the CMS regulation (not the statute) clinical
How are outpatient labs paid?
Outpatient clinical laboratory services are paid based on a fee schedule in accordance with Section 1833 (h) of the Social Security Act. Payment is the lesser of the amount billed, the local fee for a geographic area, or a national limit. In accordance with the statute, the national limits are set at a percent of the median of all local fee schedule amounts for each laboratory test code. Each year, fees are updated for inflation based on the percentage change in the Consumer Price Index. However, legislation by Congress can modify the update to the fees. Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule.
How much is the reduction for CY 2021?
There is a 0.0 percent reduction for CY 2021, and payment may not be reduced by more than 15 percent for CYs 2022 through 2024. Effective January 1, 2018, CLFS rates will be based on weighted median private payor rates as required by the Protecting Access to Medicare Act (PAMA) of 2014.
When will CLFS rates be based on PAMA?
Effective January 1, 2018, CLFS rates will be based on weighted median private payor rates as required by the Protecting Access to Medicare Act (PAMA) of 2014. For more details, visit PAMA Regulations. CMS held calls on the final rule and data reporting. For links to the slide presentations, audio recordings, and written transcripts, see CMS Sponsored Events.
How much does a cervical smear cost?
Also, for a cervical or vaginal smear test (pap smear), the fee cannot be less than a national minimum payment amount, initially established at $14.60 and updated each year for inflation.
How long is the data reporting cycle for CDLTs?
After the next data reporting period, there is a three-year data reporting cycle for CDLTs that are not ADLTs, (that is 2025, 2028, etc.).
When is the next data reporting period for CDLTs?
The next data reporting period of January 1, 2022 through March 31, 2022, will be based on the original data collection period of January 1, 2019 through June 30, 2019. After the next data reporting period, there is a three-year data reporting cycle for CDLTs that are not ADLTs, (that is 2025, 2028, etc.).
Do co-pays apply to lab fees?
Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule. Each year, new laboratory test codes are added to the clinical laboratory fee schedule and corresponding fees are developed in response to a public comment process.
How are outpatient labs paid?
Outpatient clinical laboratory services are paid based on a fee schedule in accordance with Section 1833 (h) of the Social Security Act. Payment is the lesser of the amount billed, the local fee for a geographic area, or a national limit. In accordance with the statute, the national limits are set at a percent of the median of all local fee schedule amounts for each laboratory test code. Each year, fees are updated for inflation based on the percentage change in the Consumer Price Index. However, legislation by Congress can modify the update to the fees. Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule.
When will CLFS rates be based on PAMA?
Effective January 1, 2018, CLFS rates will be based on weighted median private payor rates as required by the Protecting Access to Medicare Act (PAMA) of 2014. For more details, visit PAMA Regulations. CMS held calls on the final rule and data reporting. For links to the slide presentations, audio recordings, and written transcripts, see CMS Sponsored Events.
How much does a cervical smear cost?
Also, for a cervical or vaginal smear test (pap smear), the fee cannot be less than a national minimum payment amount, initially established at $14.60 and updated each year for inflation.
Do critical access hospitals pay for labs?
Critical access hospitals are generally paid for outpatient laboratory tests on a reasonable cost basis, instead of by the fee schedule, as long as the lab service is provided to a CAH outpatient.
How are outpatient labs paid?
Outpatient clinical laboratory services are paid based on a fee schedule in accordance with Section 1833 (h) of the Social Security Act. Payment is the lesser of the amount billed, the local fee for a geographic area, or a national limit. In accordance with the statute, the national limits are set at a percent of the median of all local fee schedule amounts for each laboratory test code. Each year, fees are updated for inflation based on the percentage change in the Consumer Price Index. However, legislation by Congress can modify the update to the fees. Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule.
How much is the reduction for CY 2021?
There is a 0.0 percent reduction for CY 2021, and payment may not be reduced by more than 15 percent for CYs 2022 through 2024. Effective January 1, 2018, CLFS rates will be based on weighted median private payor rates as required by the Protecting Access to Medicare Act (PAMA) of 2014.
When will CLFS rates be based on PAMA?
Effective January 1, 2018, CLFS rates will be based on weighted median private payor rates as required by the Protecting Access to Medicare Act (PAMA) of 2014. For more details, visit PAMA Regulations. CMS held calls on the final rule and data reporting. For links to the slide presentations, audio recordings, and written transcripts, see CMS Sponsored Events.
How much does a cervical smear cost?
Also, for a cervical or vaginal smear test (pap smear), the fee cannot be less than a national minimum payment amount, initially established at $14.60 and updated each year for inflation.
How long is the data reporting cycle for CDLTs?
After the next data reporting period, there is a three-year data reporting cycle for CDLTs that are not ADLTs, (that is 2025, 2028, etc.).
When is the next data reporting period for CDLTs?
The next data reporting period of January 1, 2022 through March 31, 2022, will be based on the original data collection period of January 1, 2019 through June 30, 2019. After the next data reporting period, there is a three-year data reporting cycle for CDLTs that are not ADLTs, (that is 2025, 2028, etc.).
Do co-pays apply to lab fees?
Co-payments and deductibles do not apply to services paid under the Medicare clinical laboratory fee schedule. Each year, new laboratory test codes are added to the clinical laboratory fee schedule and corresponding fees are developed in response to a public comment process.